Water molecules
Describe the water molecule:
A water molecule consists of 2 hydrogen atoms bonded to an oxygen atom, and its overall structure is bent.
Water is adhesive because water molecules tend to attract to other
substances.
Water has high surface tension because water is extremely cohesive, and it is difficult to break the surface of water.
Water is good at capillary action because its cohesive and adhesive.
Water has high heat capacity because the amount of heat energy required to increase water's temperature is relatively high.
Ice is less dense than liquid water because water expands slightly upon freezing.
Water is "Universal Solvent" because it can dissolve many substances
Why do we need to drink water?
Water is essential to our health. It is necessary for many of our body's functions, such as delivering nutrition to cells, removing waste, protecting bones and organs, and maintaining body temperature.
Benefits for drinking water
1. Increases Energy & Relieves Fatigue
2. Promotes Weight Loss
3. Flushes Out Toxins
4. Improves Skin Complexion
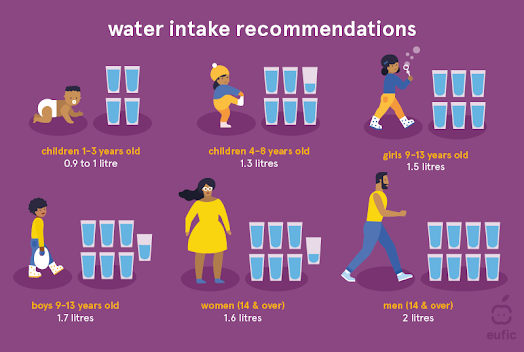
How much water should you drink daily?
Children aged 4 to 8 require five cups every day. Older kids should drink seven to eight glasses of water a day. Teenagers need 8–11 cups daily, adult females require 9 cups, while adult males needed 13 cups.
LAB REPORT:
Describe briefly the water experiments you did.
Properties of water station lab: To gain more knowledge about water and its properties, we tested water interaction with itself and other substances, and waters ability and inability to dissolve substances









seriously obsessed
ReplyDeletewowowowo
ReplyDeletecryingggg
ReplyDeletewowowow
ReplyDeleteWOWW
ReplyDeleteI LOVE THIS
ReplyDeleteso informative
ReplyDeleteQOOWOOWO
ReplyDeleteI LOVEEEE
ReplyDeleteNICEEEE
ReplyDeleteIm in love !!!
ReplyDeleteThis is really so informative! Thank you!
ReplyDeleteI'm obsessed!!!
ReplyDeleteLoveee
ReplyDeleteWOWW
ReplyDeleteNicee!! so informative, definitely will be studying from it later.
ReplyDeleteThe images are awesome! They really help me visualize your explanations!
ReplyDeleteAwesome!!
ReplyDelete